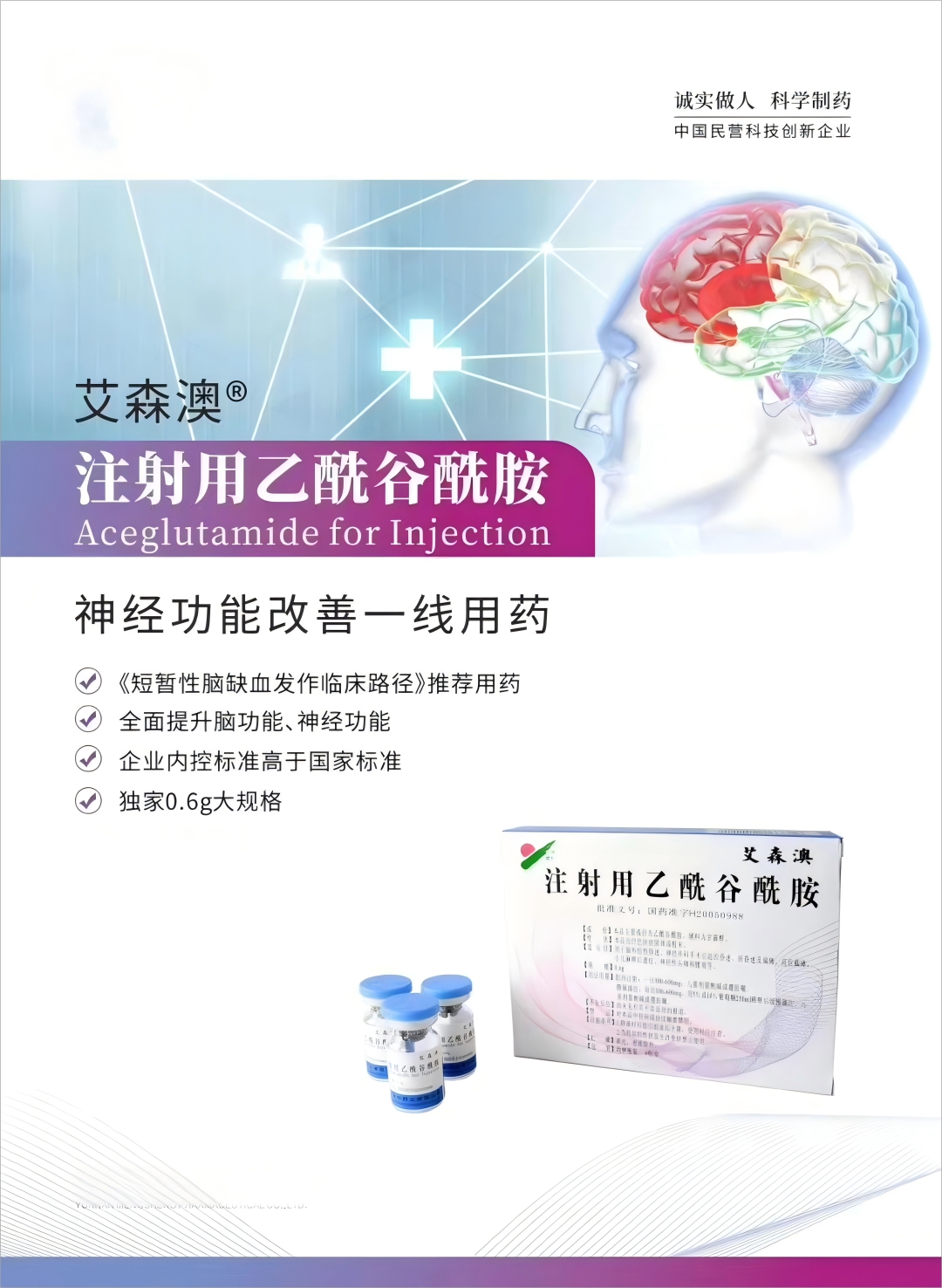
描述
[Ingredients]The main component of this product is acetylglutamine,The auxiliary material is mannitol
[Chemical name]: N2-acetyl-L-glutamine
[chemical structural formula]:
[Molecular formula]: C7H12N2O4
[ Molecular weight]: 188.18
[Character] This product is a white block solid or powder.
[indication] It is used for traumatic coma, neurosurgery caused by coma, liver coma and hemiplegia, high paraplegia, polio sequelae, nerve headache and lumbpain, etc.
[Specification] (1)0.3g (2)0.6g
[Usage and dosage]
Intramuscular injection: 300-600mg per day; dose reduction in children or as advised.
Injection: 300-600mg each time, diluted slowly in 5% or 10% 250ml glucose. Dose reduction in children or as prescribed.
[untoward effect]
The following adverse reactions / adverse events (unknown incidence):
Systemic reactions: shivering, chills, fever, fatigue, etc;
Skin and subcutaneous tissue: flushing, rash, itching, erythema, sweating, etc.;
Digestive system: nausea, vomiting, abdominal discomfort, abdominal pain, diarrhea, abdominal distension, transaminase elevation, etc.;
neurological and mental reactions: dizziness, headache, head discomfort, tremor, itching, depression, sensation, irritability, anxiety, insomnia, etc.;
Cardiovascular system: chest discomfort, chest pain, palpitations, cyanosis, elevated blood pressure, decreased blood pressure, hypotension, etc.;
Respiratory system: dyspnea, shortness of breath, suffocation, etc.;
immune system: anaphylactic reaction, anaphylactic shock, etc.;
others: phlebitis, injection site reaction (pain, induration, swelling), etc.
[Points for attention]
- Quiet drops may cause blood pressure to drop, which should be noted when using.
- Prohibition of usewhen their traits change.
[Drug use for pregnant women and lactating women] This experiment was not conducted and there is no reliable reference.
[Children’s Medication]
The use of this product by children should be reduced or follow the doctor’s advice as appropriate.
[Aged medication]
There is no study data on the medication of elderly patients, so refer to [usage and dosage] and other items.
[drug interaction]
This experiment was not performed and no reliable references exist.
[overdose]
There are no research data and literature reports on drug overdose. Once an overdose occurs, symptomatic and supportive treatment should be given.
[Pharmacological toxicology]
Acetylglutamine is an acetyl compound of glutamamide that decomposes into glutamate and γ -aminobutyric acid (GABA) after passing through the blood-cerebrospinal fluid barrier. Glutamic acid is involved in information transmission in the central nervous system.γ -Aminobutyric acid can antagonize the excitability of glutamate, improve nerve cell metabolism, maintain nerve stress ability and reduce the effect of blood ammonia, and improve brain function.
[pharmacokinetics]
This product is widely distributed in the body, with high concentration in the brain, liver and kidney, and can pass through the blood-cerebrospinal fluid barrier. In renal tubular cells decompose ammonia into acetylglutamate. Ammonia is secreted and excreted through the renal tubules, and acetylglutamate is absorbed to participate in the metabolism in the body.
[Store Hidden] shading, closed preservation.
[Packaging] Vial box 6 vials / box
[expiry date] 24 months
[Implementation standard]
China Food and Drug Administration National Drug Standard YBH12832005-2014Z
[Approval Document No.] (1) National drug approval word H20050987
(2)Chinese drug approval word H20050988


商品評價
目前沒有評價。